Guardians of the Gut: Unveiling the Marvels of Your Microbiome
The Hall lab proudly presents “Guardians of the Gut,” a remarkable journey through the captivating world of your microbiome. In collaboration with local artists and programmers, particularly Dr Jenni Rant from the SAW Trust, we’ve crafted an extraordinary interactive exhibit that takes you on a fascinating tour of the human gut.
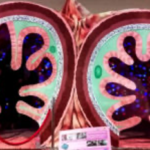
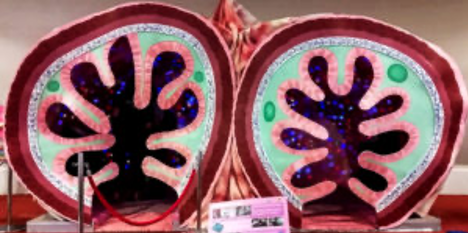
Imagine stepping into a giant walk-through model of the human gut, enhanced with a mesmerising display of 1,500 LED lights meticulously programmed to mimic the microbiome. Our passionate Hall lab guides will accompany you on this unique adventure.
During your exploration, you’ll discover the intricacies of early life microbial colonisation, including the differences between C-section and natural delivery. Learn how breastfeeding stimulates the growth of beneficial Bifidobacterium, how specific diets impact different microbial communities as they digest food, and how antibiotics can alter our resident microbes.
Your journey culminates with a showcase of the ground-breaking therapies and approaches developed by the Hall lab and others. These innovations are designed to nurture healthy early life microbial ecosystems, contributing to both overall well-being and the treatment of diseases.
‘Guardians of the Gut’ has been showcased at prestigious events, including the Royal Society Summer Exhibition, the Norwich Science Festival, and the Latitude Music Festival. To learn more about how the ‘Gut’ was brought to life with the help of our local Norwich community, click [here].
Excitingly, we are currently planning and seeking funding for a rebooted Guardians of the Gut #2, so keep eye on our Twitter/X and website for updates on where ‘Guardians of the Gut’ will make its next appearance.
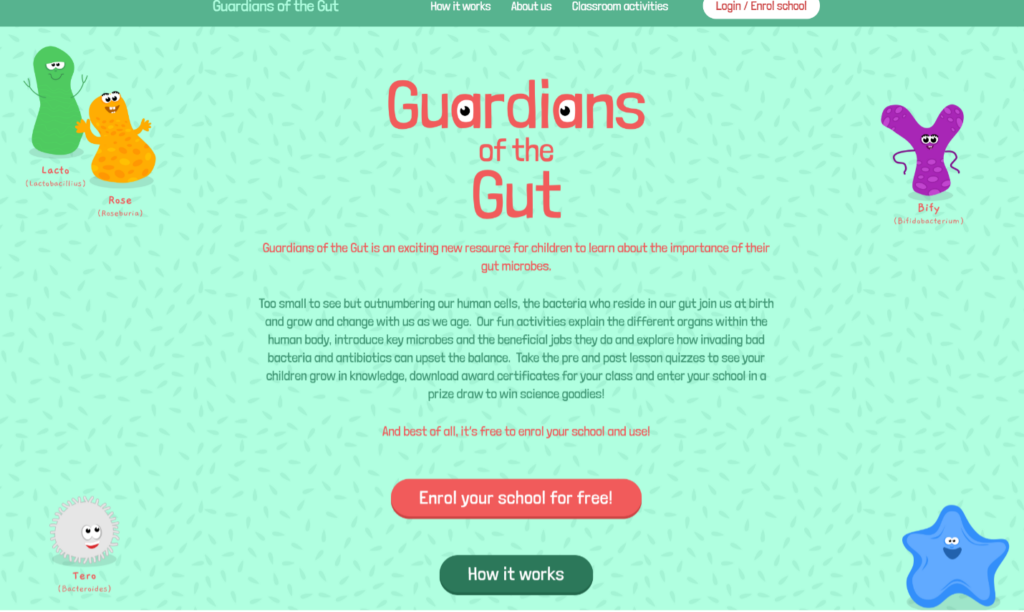
Building on our physical ‘Guardians of the Gut’ exhibit, we have also developed an online teaching resource – https://guardiansofthegut.org/, which is designed to help children understand the significance of their gut microbes. Our interactive activities provide insights into the various organs of the human body, introduce essential microbes and their beneficial roles, and delve into how harmful bacteria and antibiotics can disrupt this delicate balance. You can track your children’s learning progress with pre and post-lesson quizzes, and even download achievement certificates for your class. By enrolling your school, you also have the opportunity to participate in a prize draw for exciting science-related prizes! The best part? It’s entirely free to enrol your school and make use of this invaluable resource!
We’re incredibly grateful for the support that has made our different ‘Guardians of the Gut themed exhibits and activities a reality. This engaging project has received funding from the Wellcome Trust Engagement Fellowship, the Microbiology Society Microbiology in Society Award 2018, and the Biotechnology and Biological Sciences Research Council (BBSRC), which strategically supports the Quadram Institute.